Mass spectrometry: Enabling precision in the production of climate-friendly e-Fuels
As climate data continue to confirm record-breaking global temperatures, the pressure to decarbonize energy systems intensifies. The 2024 global average surface temperature exceeded pre-industrial levels by approximately 1.55°C, underscoring the urgency of achieving the 2030 and 2050 net-zero targets. In this context, electro-fuels (e-fuels), synthetic hydrocarbons derived from renewable energy, are gaining attention as viable substitutes for fossil-derived fuels.
Unlike hydrogen or electricity, e-fuels are drop-in replacements compatible with existing internal combustion engines, pipelines and refineries. Their production, however, is chemically and analytically complex, integrating carbon capture, water electrolysis and catalytic synthesis via the Fischer–Tropsch (FT) process. Across these interconnected stages, process mass spectrometry (MS) provides the analytical capability to monitor, optimize and validate each step in real time.
The analytical core: Magnetic sector mass spectrometry. Process MS has been used in the energy and chemical sectors for decades, but its versatility and robustness make it uniquely suited for e-fuel production. A scanning magnetic sector mass spectrometer separates ions according to their mass-to-charge (m/z) ratio in a magnetic field. The ion source, typically operating under high vacuum (~10⁻⁶ mbar), ionizes gaseous samples via electron impact (EI), those ions are accelerated into a variable magnetic field where they are separated according to their mass to charge ratio then quantified using a faraday detector. The mass spectrum recorded is characteristic of the sample’s composition. A schematic of a scanning magnetic sector MS is shown in FIG. 1.
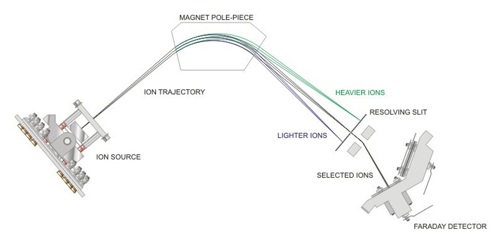
FIG. 1. The scanning magnetic sector MS.
Magnetic sector instruments remain preferred in process environments due to their wide dynamic range from parts per million (ppm) to 100% concentration, negligible mass discrimination and near-infinite detector durability. For higher sensitivity requirements, secondary electron multipliers (SEMs) or microchannel plates (MCPs) are employed, achieving part per billion (ppb)-level detection.
In multi-stream installations, a multi-stream sampler allows rapid switching, often from 30 or more sample points without interrupting flow. This architecture enables simultaneous monitoring of complex process networks such as carbon capture, electrolysis and FT synthesis loops.
Carbon capture and direct air capture (DAC) applications. The e-fuels value chain begins with carbon dioxide (CO2) capture, which supplies carbon for conversion into syngas (CO + H2). DAC systems remove CO2 from ambient air using either solvent-based absorption (amines, hydroxides) or solid sorbent-based adsorption (metal-organic frameworks or MOFs, zeolites). Given atmospheric CO2 concentrations near 400 ppm, DAC operations demand precise process control to ensure efficiency and minimize energy penalties.
MS offers a rapid, multi-component analytical capability that surpasses gas chromatography (GC) in cycle time. For example, while GC may require ~60 minutes for the complete analysis cycle of ~10 streams, MS achieves equivalent compositional quantification in under 3 minutes. Typical DAC process streams contain CO2 (80% - 98%), N2 (0% - 15%), O2 (0 % – 5 %) and H2O (0% – 6%).
An important metric for DAC operation is moisture content. H₂O present in captured CO₂ can combine with trace sulfur dioxide (SO2), nitrogen dioxide (NO2) or hydrogen sulfide (H2S) to form corrosive acids, endangering downstream piping and sequestration infrastructure. MS directly quantifies H2O and other trace components in the ppm and percent range, a measurement that is very challenging for GC, providing a robust early-warning system against corrosion risk.
Although DAC deployment remains nascent, with roughly 27 large-scale plants operating globally, MS analytics will be indispensable as capacity scales toward the 2030 goal of removing mega metric tons of CO2 annually.
Green H2 and electrolyzer diagnostics. H2 serves as the principal reductant in e-fuel synthesis. Its environmental profile, however, depends on production methodology. Green H2 is produced through water electrolysis powered by renewable electricity, yielding H2 and O2 without CO2 emissions.
Process MS contributes at both development and operational levels. For example, experiments performed on membrane-free electrolyzers by Clean Power Hydrogen (CPH2) demonstrated how magnetic sector MS enables real-time evaluation of gas purity and phase separation performance. FIG. 2 shows MS data from the cryogenic stage downstream of the electrolyzer during a fault condition, the hydrogen concentration is only ~70%, the limitation is caused by excess liquid oxygen, when this is lifted for a brief period the hydrogen content rises quickly. This real-time data from the MS enables rapid fault diagnosis and subsequent improvements to system design.
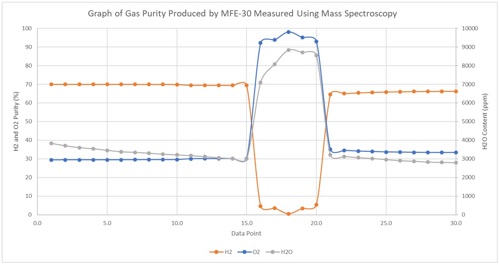
FIG. 2. Data downstream of the cryogenic system in fault condition observed by MS.
Typical MS performance data show detection limits of ≤ 0.02% and standard deviations below 0.05% for both H2 and O2 streams. The ability to monitor multiple sampling points, electrolyzer stacks, outlet pipelines and purification units, provides a comprehensive view of system behavior. Additionally, inert nitrogen purging of RMS and rotary pump assemblies ensure that mixed H2/O2 sampling remains within safe flammability limits.
Isotopic fingerprinting: Assessing H2 provenance. One important analytical study of green hydrogen involves isotope ratio determination for verifying the green provenance of H2. The natural abundance of hydrogen isotopes is approximately:
- 985% of H2 atoms are protium (H) which has no neutrons.
- 015% of H2 atoms are deuterium which has one neutron.
Studies of these isotopic ratios of H2 from differing sources have shown that fossil produced H2 has a higher deuterium content as observed by the H2 to HD ratio.
Magnetic sector MS, operating in dual-resolution mode, can resolve these low-mass ions with exceptional accuracy. The system employs separate high and low resolving slits in front of the detector, automatically switching via software control depending on mass number. Concentrations of HD near 150 ppm can be measured with a precision of ±5 ppm, sufficient to discriminate between fossil-based and renewable H2 sources. FIG. 3 shows the spectrum of a H2 sample where the HD concentration at mass 3 is approximately 1,000 ppm of the total H2 in the sample verifying correctly that this H2 is of fossil based origin.
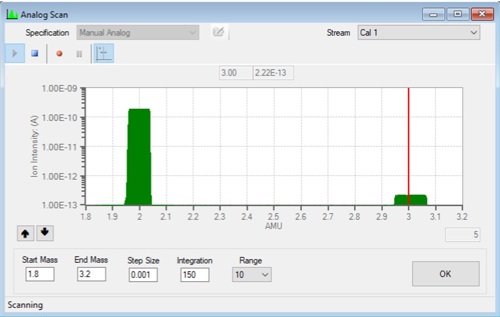
FIG. 3. The spectrum of fossil based H2 showing the large content of HD.
Such isotopic analysis could form the foundation for traceable certification of H2 origin, supporting the development of carbon accounting standards and renewable fuel guarantees of origin.
From syngas to synthetic fuels: The FT process. The synthesis of e-fuels culminates in the FT process, where CO and H2 are catalytically converted to hydrocarbons under elevated pressures (20 bar–40 bar) and temperatures (200°C–350°C). The catalyst, typically iron or cobalt, facilitates chain growth, producing paraffinic hydrocarbons suitable for refining into gasoline, diesel or sustainable aviation fuel (SAF).
MS is deeply integrated into FT operations. It quantifies syngas feed composition and monitors effluent streams containing a diverse mixture of hydrocarbons (C1–C6+), CO2, CO and inert gases. For instance, a representative FT reactor outlet might include 15% CO, 10% CO2, 5.5% methane (CH4) and numerous trace olefins and paraffins. The magnetic sector MS performs such analyses in < 20 sec, enabling near-continuous process feedback for catalyst performance optimization.
Recent pilot projects, such as those at a refinery in Bilbao, Spain, apply this analytical methodology to produce SAF via renewable H₂ and captured CO₂. These efforts align with the European Union mandate requiring that at least 70% of jet fuel supplied at EU airports be sustainable by 2050—a policy target that will demand both scale and analytical rigor.
Process MS as an enabling technology for decarbonization. Across the e-fuel production chain, the analytical requirements are formidable: multiple gas phases, rapid process transients and species ranging from major components to ppm-level impurities. Process MS, particularly of the scanning magnetic sector type, offers the combination of speed, stability and dynamic range necessary to meet these challenges.
By delivering quantitative, real-time data on critical species, from CO2 and H2O in DAC systems to H2, O2 and hydrocarbon intermediates in FT reactors, MS instruments enable closed-loop process control and performance optimization. Moreover, innovations such as isotopic fingerprinting and multi-stream sampling extend MS capabilities beyond routine quantification to full lifecycle traceability.
Takeaway. e-fuels represent a pragmatic path toward decarbonization, providing a means to recycle atmospheric carbon into high-energy liquid fuels compatible with current infrastructure. Their success, however, hinges on precise process understanding and control.
MS stands as an enabler in this transition. It unites high-resolution chemical insight with the responsiveness required for modern process systems. As the global energy landscape evolves toward renewable integration and carbon circularity, process MS will continue to play a pivotal role, offering the measurement fidelity that underpins cleaner, more efficient fuel synthesis.







Comments